Our Platform Technologies
Each of our platforms is based on science in-licensed from world-leading researchers. These platforms have the potential to reverse and prevent multiple aging-related diseases, each with critical unmet medical needs.
Partial Epigenetic Reprogramming Platform
The epigenome consists of chemical modifications of DNA and DNA binding proteins that turn genes on and off. The epigenome drifts with age, leading to dysregulated gene expression.
Our epigenetic reprogramming platform reprograms the epigenome of older animals to resemble the epigenome of younger animals via expression of three Yamanaka factors [OCT4, SOX2, and KLF4 (OSK)]. Intravitreal injection of our OSK therapy increased nerve regeneration following crush injury of the optic nerve, restored vision in a mouse glaucoma model, significantly improved vision in naturally aged mice, and demonstrated restoration of visual function and increased nerve axon survival in a non-human primate (NHP) model of non-arteritic anterior ischemic optic neuropathy (NAION). No adverse safety findings were seen in mice 15 months after systemic delivery of the OSK therapy.
Mechanism of Action

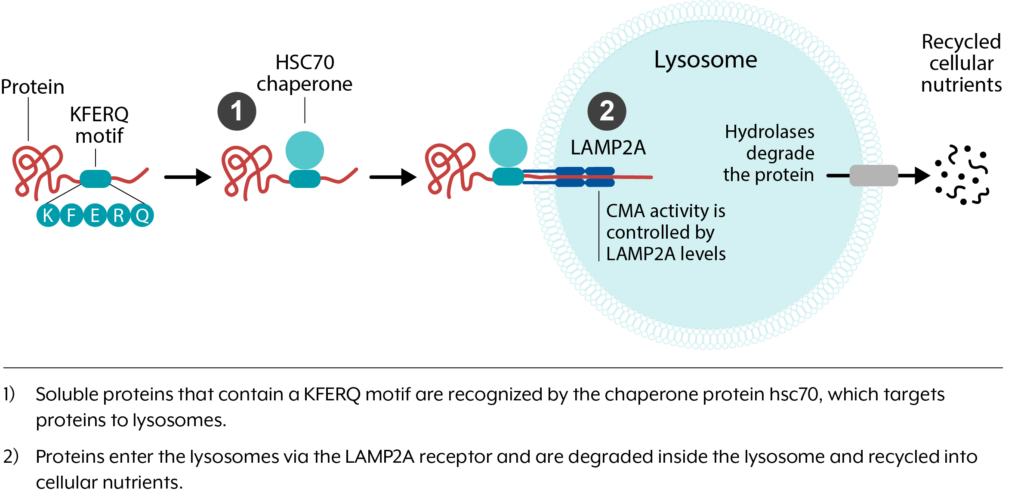
Chaperone-Mediated Autophagy Platform
Chaperone-mediated autophagy (CMA) is a process by which unwanted proteins are degraded in cells. CMA activity declines during aging due to a reduced expression of a protein called LAMP2A, which leads to the accumulation of insoluble protein aggregates that disrupt cellular function.
We have identified novel small molecule oral compounds that increase the expression of multiple proteins in the CMA pathway, including LAMP2A, and thereby increase CMA activity. Our CMA activator compounds have been shown to have efficacy in preclinical models of frontotemporal dementia, Alzheimer’s disease, and retinal degeneration.
Mechanism of Action